Approximate Read Time: 20 minutes
“Healing isn’t about control. It is about coordination. Exosome therapy simply reminds the body how to communicate.”
What You Will Learn
- How exosome therapy enhances tissue healing and performance recovery for athletes and active adults.
- Why exosomes are emerging as a cell-free alternative to PRP, BMAC, and stem cells.
- The clinical translation of exosome therapy for knee arthritis, tendinopathy, and muscle injuries.
What’s Next in Regenerative Sports Medicine
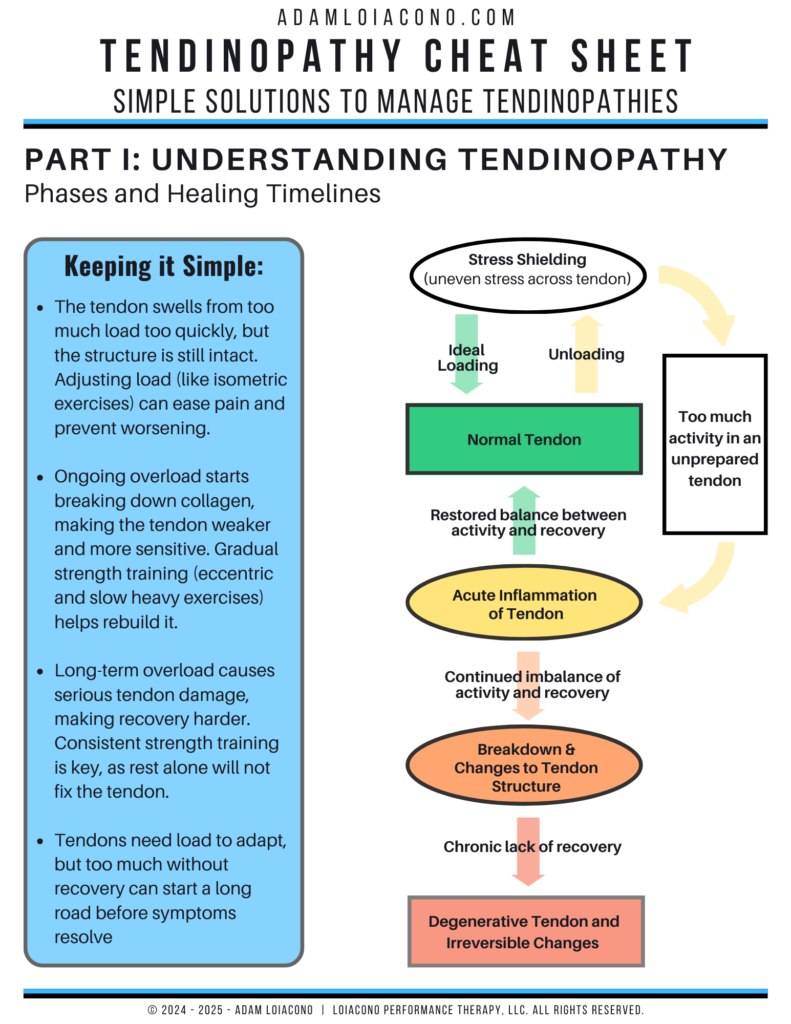
In sports medicine, the best outcomes come from the right intervention, at the right time, for the right tissue. For decades, we’ve relied on traditional orthobiologics like platelet-rich plasma (PRP) and bone marrow aspirate concentrate (BMAC) to stimulate healing. These tools helped us move beyond symptom management and closer to true biological repair. But each has its limits such as inconsistent products, variable potency, and sometimes unpredictable inflammation.
Exosomes are the microscopic messengers that stem cells use to communicate healing. Rather than transplanting live cells, exosome therapy delivers their bioactive signals (the cytokines, growth factors, and microRNAs) direct tissue repair.
Across recent studies, exosomes have shown measurable impact in cartilage repair, tendon healing, and muscle regeneration (Wu et al., 2022; Xavier et al., 2023; Iyer et al., 2023). Preclinical models demonstrate improved collagen alignment, faster functional recovery, and reduced inflammation compared to PRP or cell-based injections.
But perhaps most importantly, exosome therapy is translating from the lab to the clinic and helping bridge the gap between regenerative science and real-world performance care.
Science Background: What Are Exosomes?
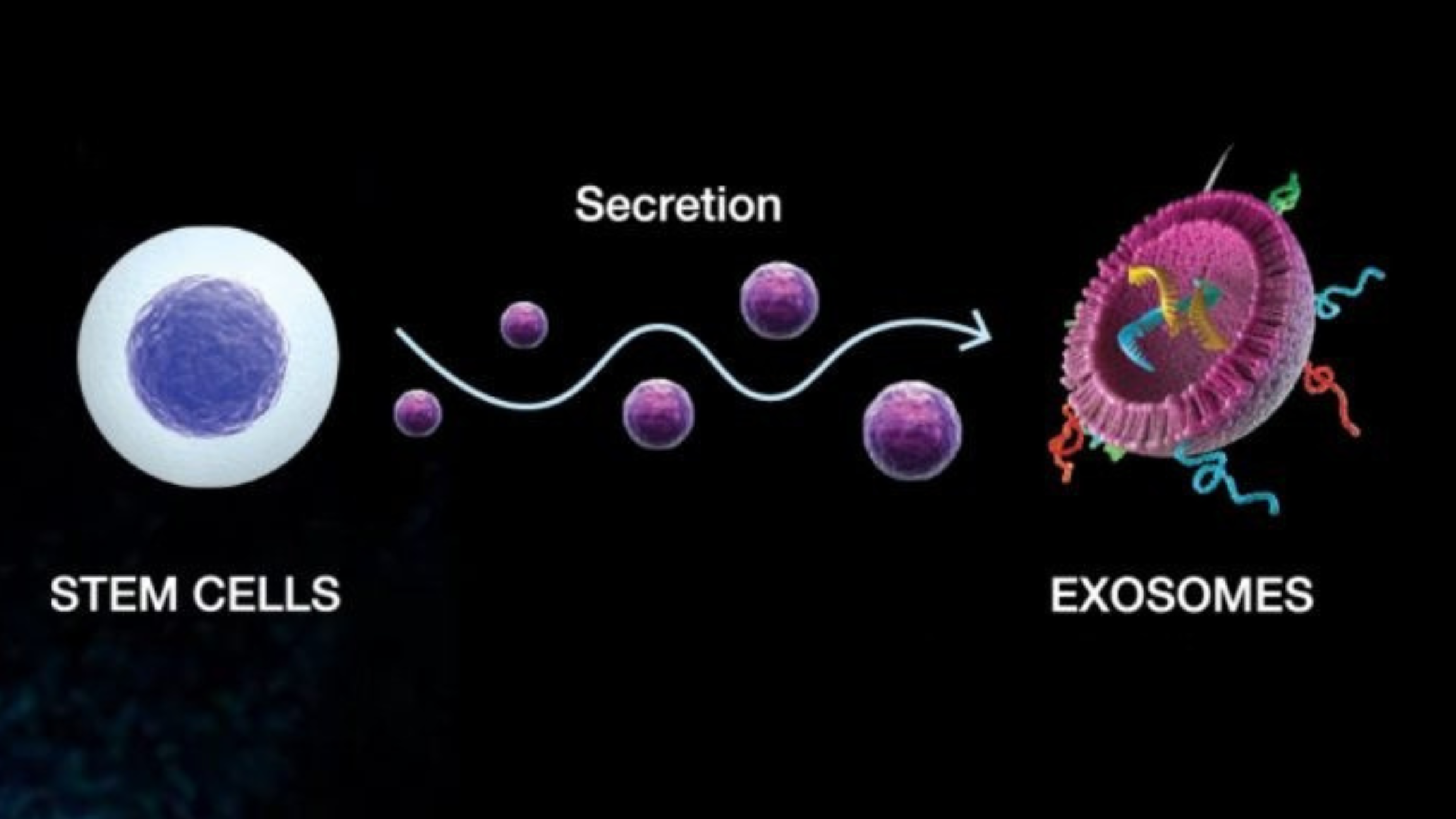
Exosomes are extracellular vesicles, nanosized “packets” released by nearly every cell type. Their cargo includes messenger RNA, microRNAs, proteins, and lipids that regulate how nearby or distant cells behave. In simple terms, they are the body’s natural communication network for healing. Think of them as text messages.
Imagine a construction site. Stem cells are your general contractor. Exosomes are the text messages the contractor sends to sub-contractors. Peptides are the construction materials used to complete the job.
In regenerative medicine, most clinical-grade exosomes are isolated from mesenchymal stem cells (MSCs) often derived from umbilical cord tissue, Wharton’s Jelly, or bone marrow. These MSC-derived exosomes carry a molecular signature rich in anti-inflammatory and pro-regenerative factor that orchestrate processes like angiogenesis, collagen synthesis, and immune modulation
The advantage? Exosomes provide the therapeutic benefits of stem cells without the logistical or ethical challenges of handling live cells:
- No need for cell culture or expansion.
- Minimal immune rejection risk.
- Greater consistency between doses.
- Easier storage and transport.
These properties make exosomes ideal for use in sports and orthopedic medicine, where precision, reproducibility, and improved recovery timelines are desired.
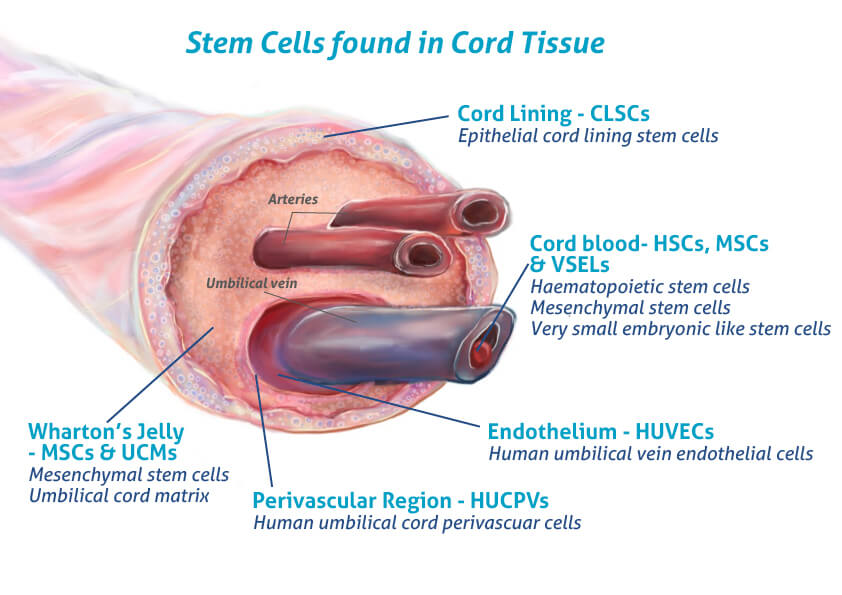
Mechanism of Action: How Exosomes Support Tissue Repair
When injected into damaged tissue, whether a degenerated tendon or arthritic joint, exosomes act as biological coordinators. They don’t rebuild the tissue directly; instead, they signal the body’s own cells to repair, regenerate, and restore homeostasis.
At the cellular level, MSC-derived exosomes influence three main mechanisms:
- Inflammation Modulation
Exosomes suppress pro-inflammatory cytokines and upregulate anti-inflammatory mediators. This helps calm the “fire” of acute injury without blunting necessary early immune responses (Cosenza et al., 2017; Wu et al., 2022). - Cellular Regeneration and Remodeling
By delivering growth-promoting microRNAs, exosomes stimulate chondrocyte and fibroblast activity and regulate extracellular matrix turnover. This leads to improved collagen production and suppression of damaging enzymes (Xavier et al., 2023) - Angiogenesis and Mitochondrial Repair
Exosomes enhance blood vessel formation through VEGF signaling and restore mitochondrial function in injured muscle fibers.
Together, these effects create a healing microenvironment that can accelerate recovery and support long-term tissue resilience.
Exosome Therapy and Athlete Care
Regenerative medicine has always pursued a single goal, restore structure and function so the body can perform as if it were never injured. For athletes, that means faster recovery, stronger tissue, and less downtime. Exosome therapy is emerging as a legitimate adjunct for achieving all three.
In the following sections, we’ll explore how exosomes are being used in knee osteoarthritis, post-operative healing, and chronic tendinopathy, the very injuries that define modern sports medicine.
Knee Osteoarthritis: Cartilage Repair and Pain Reduction
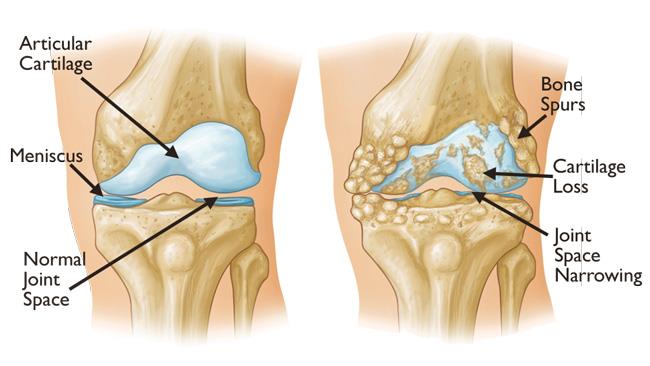
Knee osteoarthritis (OA) is no longer a diagnosis reserved for older adults. High-volume training, joint trauma, and repetitive stress can accelerate cartilage wear even in athletes under thirty. Traditional options (corticosteroids, hyaluronic acid, or PRP) may reduce pain but have mixed outcomes in terms of tissue regeneration.
MSC-derived exosomes promote activity of the very cells that can stimulate cartilage growth. These cells, chondrocytes, become more active with the use of exosome therapy. (Wu et al., 2022; Xavier et al., 2023) In animal and early human models, intra-articular injections produced smoother cartilage surfaces, improved biomechanics, and lower pain scores compared to PRP or saline controls.
Unlike total knee arthroplasty or microdrilling procedures that create fibrocartilage, exosomes appear to support hyaline-like cartilage regeneration, closer in structure to native tissue. Meaning, exosomes are stimulating the right type of cartilage in comparison to these surgical techniques stimulating the incorrect type.
For active adults and professional athletes, this means biological repair without surgical downtime, a compelling alternative when joint replacement is too extreme and cortisone too temporary.
2. Post-Operative Recovery: ACL and Achilles Repair
Post-surgical recovery is a biological paradox: the procedure fixes the structure, but the surrounding environment often remains inflamed, hypoxic, and slow to remodel. Here, exosomes function as a recovery accelerator.
In both ACL reconstruction and Achilles tendon repair, the early healing environment is dominated by inflammation and scar formation. MSC-derived or umbilical-cord exosomes introduced into graft or tendon sites downregulate pro-fibrotic genes and upregulate regenerative pathways (Wu et al., 2022; Liu et al., 2021).
Post-operative athletes who are treated with exosome therapy have the potential for:
- Faster reduction in post-surgical swelling and effusion
- Accelerated collagen organization around the graft site
- Improved load tolerance and early return-to-movement milestones
- Fewer complications related to graft fibrosis or stiffness
The rationale mirrors what we see clinically in strength training: load drives adaptation, but only when inflammation is controlled. By modulating inflammation rather than suppressing it, exosomes help rebuild the environment athletes need for progressive loading and safe return to play.
FREE DOWNLOAD
Want to Learn How to Optimize Recovery After Stem Cell Treatment?
3. Chronic Tendinopathy: Restoring Load Capacity
Chronic tendinopathies like patellar, Achilles, proximal hamstring, or elbow are among the most frustrating injuries for both athletes and clinicians. They resist time, load management, and even surgery. The challenge lies not just in structure but in biology: tenocytes trapped in a cycle of degeneration, collagen disorganization, and failed healing.
Exosomes appear to reactivate dormant tenocytes and promote type I collagen synthesis (Ni et al., 2020; Zhang et al., 2021). In animal models of tendon-bone healing, exosome injections enhanced integration at the repair site and improved tensile strength compared with PRP.
For athletes, this can translate to:
- Enhanced tendon stiffness and elasticity
- Improved tolerance to eccentric and plyometric loading
- Decreased recurrence of symptoms
In the continuum of tendon rehab from isometrics to heavy slow resistance to plyometrics, exosome therapy supports the biological recovery that allows mechanical loading to do its job.
Efficacy of Exosome Therapy by Condition
| Condition | Key Mechanism | Observed Outcomes | Improvement Timeline | Evidence Level |
|---|---|---|---|---|
| Knee Osteoarthritis | Reduces inflammation, stimulates cartilage regeneration | Less pain, better function, MRI shows cartilage repair | 4–6 weeks onset; sustained 6–12 months | Human Phase I/II trials; clinical use abroad |
| ACL / Achilles Post-Op | Promotes angiogenesis, collagen organization | Faster graft healing, less swelling, earlier mobility | 2–8 weeks tissue response | Preclinical + early human data |
| Chronic Tendinopathy | Activates tenocytes, regulates collagen synthesis | Pain reduction, greater tendon stiffness, better function | 2–4 weeks pain relief; 2–3 months tissue gains | Animal + case series |
| Muscle Strain Injury | Supports myogenesis, limits fibrosis | Faster strength recovery, healthier muscle histology | 1–3 weeks | Preclinical (ACSM 2023) |
The Clinical Takeaway
Across these scenarios, the thread is clear: exosomes don’t replace rehab; they support it. They create the biological conditions for training and therapy to take effect faster and more completely.
Athletes who once faced a binary choice, rest or surgery, may soon have a third option: biological restoration that integrates seamlessly with performance therapy principles.
Evidence Base: What the Research Actually Shows
For a therapy to be credible in sports medicine, it must show more than promise. It must show reproducible outcomes across different models and conditions. Exosome therapy has slowly been building this evidence over recent years.
Across preclinical studies and a growing set of human trials, exosomes have demonstrated consistent effects on pain, inflammation, and tissue regeneration. The strength of evidence lies not in one landmark trial but in the convergence of results across cartilage, tendon, and muscle models.
In knee osteoarthritis, exosomes derived from mesenchymal stem cells (MSCs) have been shown to restore cartilage integrity by stimulating chondrocyte activity, up-regulating type II collagen, and suppressing pro-inflammatory markers (Xavier et al., 2023). In the same review, thirteen in-vivo studies consistently demonstrated smoother cartilage surfaces and increased International Cartilage Repair Society (ICRS) scores following intra-articular exosome treatment.
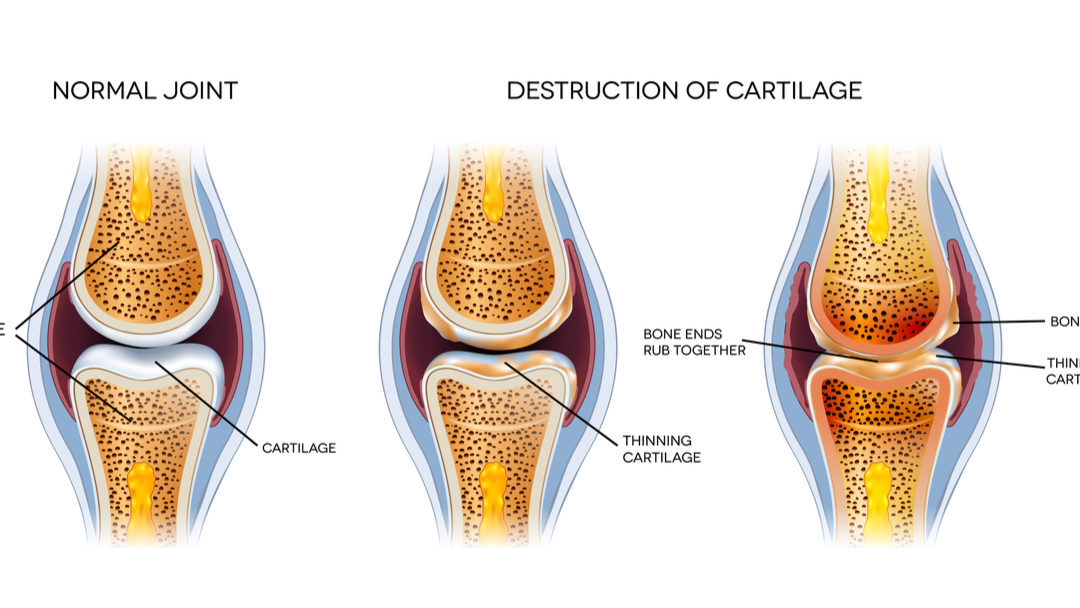
Wu et al. (2022) expanded this understanding beyond cartilage, describing exosome therapy for intervertebral disc and tendon regeneration. They identified that modified exosomes enhanced chondrocyte signaling while reducing enzymes responsible for tissue breakdown. These effects mirror the goals of sports rehabilitation: decrease destructive load, enhance constructive remodeling.
Perhaps the most compelling preclinical evidence comes from muscle injury studies. Iyer et al. (2023) reported that both platelet-rich plasma-derived and MSC-derived exosomes accelerated contractile recovery in rat tibialis anterior muscle after eccentric injury. Within fifteen days, treated muscles regained significantly greater torque and displayed higher muscle regeneration compared to saline controls (p < 0.05). This finding resonates with clinical practice, where returning eccentric strength after a strain is the final and most decisive milestone before sport reintegration.
Taken together, these results paint exosomes as more than a research novelty. They are slowly demonstrating functional regeneration.
Acronym Soup of Orthobiologics
The orthobiologic landscape is crowded with acronyms: PRP, BMAC, MSCs, and a newer player in the game, peptides. Each claims to stimulate healing, yet their biological complexity, dosing variability, and regulatory challenges differ dramatically.
Comparison of Orthobiologic Therapies
| Therapy | Primary Mechanism | Advantages | Limitations | Clinical Evidence Level |
|---|---|---|---|---|
| Exosomes | Paracrine signaling; modulate inflammation and stimulate regeneration | low adverse immune response | Limited large-scale human trials; regulatory ambiguity | Strong preclinical; early clinical (Phase I–II) (Xavier et al., 2023; Wu et al., 2022) |
| BMAC (Bone Marrow Aspirate Concentrate) | Delivers live MSCs and growth factors from autologous bone marrow | Established use; promotes osteogenesis | Cell yield declines with age; inconsistent potency | Moderate; mixed clinical outcomes (Wu et al., 2022) |
| PRP (Platelet-Rich Plasma) | Releases platelet-derived growth factors to initiate repair | Easy to prepare; widely available | Short biologic half-life; inconsistent results across devices | High usage; moderate efficacy (Wu et al., 2022) |
| Expanded MSCs | Direct differentiation + paracrine signaling | Robust regenerative signaling; multi-lineage potential | Requires cell culture, higher oversight, FDA restrictions | Preclinical + select international clinical (Wu et al., 2022; Iyer et al., 2023) |
| Peptides | Short amino acid chains that modulate healing pathways | Targeted, low cost, easy delivery | Mechanistic evidence strong; human tendon data limited | Early translational (Iyer et al., 2023) |
In simple terms, PRP and BMAC can be thought of as first-generation orthobiologics. They supply ingredients for healing but rely on the body to interpret the signal. Stem cells, exosomes and peptides are second-generation therapies: they deliver the signal itself, already encoded with instructions.
Compared to cell-based treatments, exosomes carry none of the live-cell risks. No risk of immune rejection, no tumorigenic potential, and no ethical dilemma surrounding cell sourcing. And unlike PRP, their molecular composition can be standardized, ensuring reproducible results across athletes and clinics.
In practice, exosome therapy often complements these older biologics. For example, PRP-derived exosomes can be isolated from an athlete’s own plasma, combining the convenience of PRP with the precision of exosome signaling (Iyer et al., 2023). This hybrid approach is already being used in international centers.
Safety, Regulation, and Ethical Considerations
Safety and ethics in regenerative medicine are inseparable from trust. Athletes and clinicians alike need assurance that the biologics entering their bodies are both legitimate and safe.
Exosomes are naturally cell-free, meaning they cannot replicate or form tumors. In preclinical and early clinical trials, no severe adverse events have been reported (Wu et al., 2022; Xavier et al., 2023). Common, transient effects include mild swelling or local soreness at the injection site comparable to PRP. Importantly, their acellular nature makes them immunologically quiet; they avoid the immune reactivity sometimes seen with umbilical derived stem cells.
From an ethical standpoint, the most common exosome sources are umbilical cord and Wharton’s Jelly that are harvested from consented, post-birth tissues that would otherwise be discarded. These tissues are screened for infectious disease and processed under Good Manufacturing Practice (GMP) standards, minimizing ethical or safety conflicts (Wu et al., 2022). In contrast, expanded stem cell face tighter regulation.
Regulatory oversight varies globally. In the United States, exosome therapies are not yet FDA-approved as stand-alone biologic drugs; their use falls under the “investigational” category. However, countries such as South Korea and Panama have established GMP-certified clinical programs where exosomes are integrated into orthopedic care (Xavier et al., 2023). The expectation is that the U.S. will follow once standardization of isolation and potency testing becomes more robust.
Safety and Ethical Comparison of Orthobiologics
| Therapy | Source & Manipulation | Immune Risk | Ethical | Regulatory Status (U.S.) |
|---|---|---|---|---|
| Exosomes | Cell-free vesicles, typically umbilical or MSC-derived; minimal manipulation | Very low; non-replicating | Post-birth tissue; ethically neutral | Investigational / not yet FDA-approved |
| BMAC | Autologous bone marrow aspirate; minimal manipulation | Low; autologous | Ethically acceptable | Allowed under guidance |
| PRP | Autologous blood centrifugation | Low | Ethically acceptable | Widely permitted |
| Expanded MSCs | Culture-expanded stem cells | Moderate potential with prolonged culture | Ethical sourcing required; some donor variability | Not yet FDA approved |
| Peptides | Synthetic or recombinant | Negligible | Minimal ethical concerns | Varies by compound; some OTC |
In short, exosome therapy stands out for its safety, scalability, and ethics. These qualities align well with the professional-sport environment where player welfare, anti-doping compliance, and public scrutiny are paramount.
When delivered through regulated channels, exosomes may soon become the default biologic for athletes seeking faster recovery without crossing ethical or pharmacologic boundaries.
Integration with the 3P Framework: Reframing Regeneration
The evolution of sports medicine is no longer defined by scalpels or scans. It’s defined by integration. Every new therapy, from load management to biologics, requires a framework that keeps progress grounded in principle. The 3P Model (Principles, Process, and Plans) exists for this reason: to bring order to the chaos.
Exosome therapy fits beautifully within that philosophy. It doesn’t replace training, rehabilitation, or sound clinical reasoning. It refines them. It reminds us that biology and behavior are partners, not opposites.
Principles: Coordinate, Don’t Control
The first “P” is about coherence. In performance therapy, we don’t force change. We coordinate it. The same is true in cellular regeneration. Exosomes don’t overpower the body; they coordinate healing. They send signals that remind cells how to behave: regulate inflammation, rebuild collagen, restore communication between tissues.
This parallels what every good rehab professional already knows: the body doesn’t need micromanagement. It needs the right cues, at the right time, delivered with consistency. Exosomes provide those cues at the molecular level in the same way targeted exercise provides them at the mechanical level.
We are not “fixing” the body; we are facilitating its ability to fix itself. Exosomes extend that idea into biology.
Process: Experiment and Exposure
The “Process” is where philosophy meets action. The best outcomes come from safe-to-fail experiments. Not from rigid protocols. That same experimental mindset defines the science of exosomes.
Researchers learned that unmodified exosomes were rapidly cleared from tissue (Wu et al., 2022). So they experimented pairing them with scaffolds, hydrogels, and nanoclays to improve retention. They modified microRNAs to target inflammatory genes or enhance cartilage formation (Xavier et al., 2023). Each iteration was a test–retest experiment at the molecular level, the same principle that governs every therapy session on the clinic floor.
“Slow when you can, fast when you must.” – Jim Collins
In performance rehab, exposure is gradual. We load tissue “slow when you can, fast when you must.” Biologic therapy demands the same pacing. After an injection, we don’t rush progress; we cultivate it. We expose the healing tissue to mechanical load only when it’s ready, allowing biology to rebuild capacity.
Plans: Amplify Desirables, Dampen Undesirables
The third “P” is application. Plans bring theory into real time. Whether you’re adjusting a training load or choosing a biologic, every intervention should amplify what’s desirable and dampen what’s not.
In the context of exosome therapy, desirable outcomes include reduced pain, increased tissue tolerance, and measurable performance capacity. Undesirable adaptations like prolonged inflammation, fibrosis, or stiffness are what exosomes help to neutralize.
The decision-making philosophy is simple:
- If an intervention improves movement or accelerates recovery, amplify it.
- If it disrupts coherence or adds noise to the system, dampen it.
This is how regenerative medicine avoids becoming reactionary. It becomes strategic.
Summary and Final Thought
Healing used to mean rest. Now it means regeneration. We’ve moved from ice bags and cortisone to a variety of biologics. Yet the real transformation isn’t just in technology, it’s in perspective.
Exosome therapy teaches us that healing doesn’t need to be forced; it can be guided. It proves that biology is coachable. The same way an athlete learns to move better through feedback, tissue learns to recover better through signaling.

The real story of exosomes is less about the molecule and more about the mindset. They invite us to replace the question “How do I fix this?” with “How do I help the body remember what to do?” That shift from intervention to interpretation is where the art of sports medicine lives.
In my world, that’s the heart of the 3P philosophy: principles to keep you grounded, process to keep you curious, and plans to keep you consistent. Exosomes don’t disrupt that model. They validate it. They remind us that healing is not just chemistry; it’s communication.
For the athlete, this means hope. For the clinician, it means humility. And for both, it means progress, not because science gave us a shortcut, but because we learned to listen to the body more intelligently.
Five Key Takeaways
- The future of sports medicine will be defined by coordination, not control. Exosomes represent a new language for regeneration—one where we start facilitating communication between biology, behavior, and performance.
- Exosome therapy is the next evolution of orthobiologics—a cell-free approach that delivers precise biological signals to stimulate repair, reduce inflammation, and restore tissue coherence (Wu et al., 2022; Xavier et al., 2023).
- Clinical translation is accelerating. Preclinical evidence now spans cartilage, tendon, and muscle models, with early human studies showing measurable gains in pain reduction, cartilage quality, and post-operative recovery (Iyer et al., 2023).
- Safety and ethics are favorable. Derived from post-birth umbilical tissue or autologous plasma, exosomes carry minimal immune or tumor risk and align with international GMP manufacturing standards.
- Integration beats isolation. Exosomes are not a replacement for load-based rehabilitation but a biologic amplifier that enhances tissue readiness.
FREE DOWNLOAD
Want to Learn How to Optimize Recovery After Stem Cell Treatment?
Read More Like This
Related Podcasts
References
Xavier J, Jerome W, Zaslav K, Grande D. Exosome-laden scaffolds for treatment of post-traumatic cartilage injury and osteoarthritis of the knee: A systematic review. Int J Mol Sci. 2023;24(20):15178. doi:10.3390/ijms242015178
Wu R, Li H, Sun C, et al. Exosome-based strategy for degenerative disease in orthopedics: Recent progress and perspectives. J Orthop Transl. 2022;36:8–17. doi:10.1016/j.jot.2022.05.009
Iyer SR, Scheiber AL, Yarowsky P, Henn RF III, Otsuru S, Lovering RM. Exosomes isolated from platelet-rich plasma and mesenchymal stem cells promote functional recovery after muscle injury. Presented at: American College of Sports Medicine Annual Meeting; May 2023; Denver, CO.
Ni Z, Zhou S, Li S, et al. Exosomes: Roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:10.1038/s41413-020-0100-9.
Zhang Y, Wang X, Chen J, et al. Exosomes derived from platelet-rich plasma administration in situ mediate cartilage protection in subtalar osteoarthritis. J Nanobiotechnol. 2021;20:10.1186/s12951-022-01245-8.
Liu H, Zhang M, Shi M, et al. Adipose-derived mesenchymal stromal cell-derived exosomes promote tendon healing by activating both SMAD1/5/9 and SMAD2/3 pathways. Stem Cell Res Ther. 2021;12(1):471. doi:10.1186/s13287-021-02410-w
Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cell-derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7:16214. doi:10.1038/s41598-017-15376-8
Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. doi:10.7150/thno.17133
Yue Y, Dai W, Wei Y, et al. Unlocking the potential of exosomes: A breakthrough in the theranosis of degenerative orthopaedic diseases. Front Bioeng Biotechnol. 2024;12:1377142. doi:10.3389/fbioe.2024.1377142
Zou J, Yang W, Cui W, et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon–bone healing. J Nanobiotechnol. 2023;21:284. doi:10.1186/s12951-023-01778-6
Wan R, Hussain A, Behfar A, Moran SL, Zhao C. The therapeutic potential of exosomes in soft tissue repair and regeneration. Int J Mol Sci. 2022;23(7):3869. doi:10.3390/ijms23073869
Fan WJ, Liu D, Pan LY, et al. Exosomes in osteoarthritis: Updated insights on pathogenesis, diagnosis, and treatment.Front Cell Dev Biol. 2022;10:949690. doi:10.3389/fcell.2022.949690
Zhang S, Chuah S, Lai RC, Hui J, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi:10.1016/j.biomaterials.2017.11.028
Qin B, Bao D, Liu Y, et al. Engineered exosomes: A promising strategy for tendon-bone healing. J Adv Res.2023;64:155–169. doi:10.1016/j.jare.2023.11.011
Zhang F-X, Liu P, Ding W, et al. Injectable mussel-inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials. 2021;278:121169. doi:10.1016/j.biomaterials.2021.121169
Chen J, Wang Z, Yi M, et al. Regenerative properties of bone marrow mesenchymal stem cell-derived exosomes in rotator cuff tears. J Transl Med. 2025;23:60. doi:10.1186/s12967-024-06029-2
Malekpour K, Hazrati A, Zahar M, et al. The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. Stem Cell Rev Rep. 2021;18:933–951. doi:10.1007/s12015-021-10185-z
Ni Z, Kuang L, Chen H, et al. Exosomes in cartilage repair: Mechanisms, models, and potential clinical translation.Bone Res. 2020;8:35. doi:10.1038/s41413-020-0100-9
Wu Y, Li J, Zeng Y, et al. Exosomes rewire the cartilage microenvironment in osteoarthritis: From intercellular communication to therapeutic strategies. Int J Oral Sci. 2022;14(1):10. doi:10.1038/s41368-022-00187-z





