Approximate Read Time:
“Not all hyperbaric chambers are equal—depth defines dosage, and dosage defines results.”
What You Will Learn
- How hyperbaric oxygen therapy (HBOT) can support stem cell survival and regeneration at the cellular and tissue levels.
- Why pressure, oxygen concentration, and timing are critical variables for HBOT’s synergy with mesenchymal stem cell (MSC) therapy.
- How to apply the 3P Model—Principles, Process, Plans—to integrate HBOT into performance, recovery, and regenerative protocols.
The Oxygen Paradox: Too Little, Too Much, or Just Right?
Oxygen is paradoxical—it’s both life’s fuel and its stress test. Every breath we take feeds mitochondrial respiration, the metabolic engine of human biology. Yet in excess, oxygen becomes reactive, creating free radicals that can damage the very cells it’s meant to sustain. This adverse effect is commonly known as oxidative stress.
Hyperbaric oxygen therapy (HBOT) was born from this paradox. Initially used to treat decompression sickness in divers, HBOT involves breathing 100% oxygen at pressures greater than atmospheric level—typically between 2.0 and 2.5 atmospheres absolute (ATA)—to hyper-saturate the body with oxygen.
Under this environment, oxygen dissolves directly into blood plasma, penetrating tissues far beyond red blood cell transport. The result: a temporary, measurable flood of oxygen that shifts the body’s biochemistry from survival to repair.
But the real revolution began when researchers discovered HBOT doesn’t just heal—it modulates stem cells. It mobilizes them, preserves them, and, when used in tandem with mesenchymal stem cell (MSC) therapies, amplifies their regenerative potential.
The Science of Pressure: What Happens Inside the Chamber
At sea level, air contains 21% oxygen. Inside a hyperbaric chamber, the environment changes dramatically. Pressure doubles or even triples, and oxygen concentration rises to 100%. This simple shift has profound physiological effects:
- Oxygen diffusion increases up to 20-fold, reaching tissues starved by injury or inflammation.
- Stem cell mobilization increases, as the bone marrow releases cells into circulation (Thom, 2006).
- Angiogenesis (new vessel formation) accelerates, supported by upregulation of VEGF (vascular endothelial growth factor).
- Inflammation and oxidative stress decrease through the activation of antioxidant response pathways
Collectively, these changes create an environment that supports cellular repair and the survival of transplanted or endogenous stem cells.
When paired with stem cell therapies—whether autologous bone marrow, adipose-derived, or umbilical cord mesenchymal stem cells—HBOT may serve as the oxygen-rich soil in which regenerative cells can thrive.
The Bohr Effect: Why This Matters?
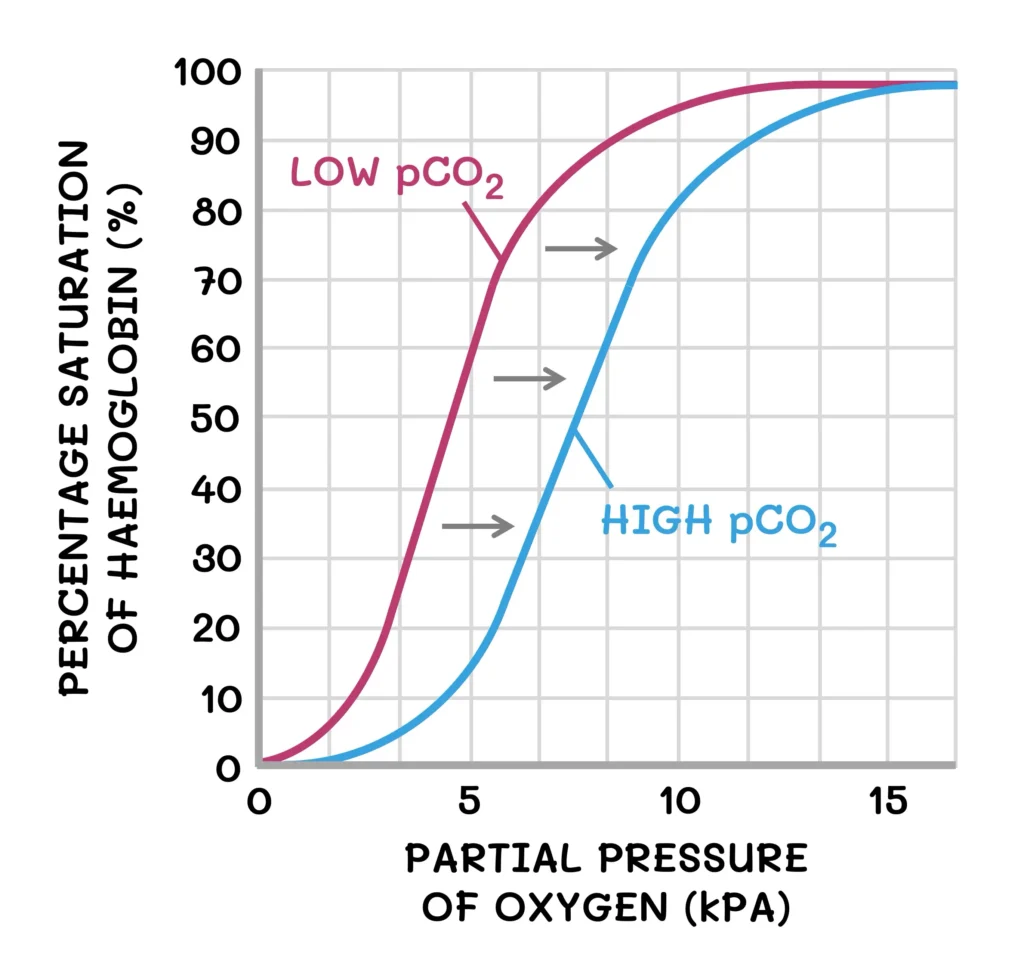
Inside a hyperbaric chamber, oxygen doesn’t just increase in quantity—it changes how it’s carried and delivered. The Bohr effect explains how hemoglobin’s grip on oxygen loosens in acidic, CO₂-rich tissues (where oxygen is needed most) and tightens in the more alkaline environment of the lungs.
During hyperbaric oxygen therapy (HBOT), this balance temporarily shifts. Breathing 100% oxygen at 2.0–2.5 ATA dramatically elevates plasma oxygen—up to 10–20 times normal levels. Oxygen transport is no longer limited by hemoglobin. Mild hyperventilation in the pressurized environment slightly reduces CO₂, raising blood pH and shifting the oxygen-hemoglobin dissociation curve leftward. In simpler terms, hemoglobin holds oxygen more tightly. Yet because plasma becomes supersaturated with dissolved oxygen, tissues still receive more oxygen through passive diffusion.
In effect, HBOT temporarily induces a leftward shift of the oxygen–hemoglobin dissociation curve by lowering CO₂ and raising pH. HBOT bypasses the constraints of the Bohr effect, overwhelming the system with readily available oxygen. The result is a temporary biochemical surplus that enhances cellular metabolism, accelerates repair, and restores tissue oxygenation even in areas starved by injury or inflammation.
| Variable | Normal Conditions | During HBOT (2.0–2.5 ATA) | Change |
|---|---|---|---|
| Blood pH | ~7.40 | ↑ ~7.45–7.50 | ⬆️ (more alkaline) |
| pCO₂ | 35–45 mmHg | ↓ ~30–35 mmHg | ⬇️ (lower CO₂) |
| pO₂ | 80–100 mmHg | ↑↑↑ 800–1500 mmHg | ⬆️⬆️⬆️ (massive rise) |
| Hemoglobin Saturation (SaO₂) | 97–98% | 100% | ⬆️ (fully saturated) |
| Plasma O₂ (dissolved) | Minimal | ↑↑ (10–20× higher) | ⬆️⬆️ (enhanced diffusion) |
| Respiratory Rate | 12–20 bpm | ↓ slightly | ⬇️ (reduced drive) |
| Inflammation | Normal baseline | ↓ | ⬇️ (anti-inflammatory) |
| O₂–Hb Curve | Normal balance | Left shift | ⬅️ (↑ affinity for O₂) |
How HBOT Enhances Stem Cell Function
Mobilization of Your Own Stem Cells
HBOT has been shown to increase your body’s own (endogenous) circulating stem cells by up to eightfold within 24 hours (Thom et al., 2006). This mobilization supports systemic regeneration and may complement stem cell therapies (exogenous).
Improved Survival
After MSC administration, tissue hypoxia and inflammation threaten cell viability. HBOT reverses local hypoxia, enhancing cell survival, differentiation, and paracrine signaling (Gardin et al., 2020).
Anti-inflammatory and Immune Regulation
MSC therapies work partly by reprogramming inflammation. HBOT strengthens this effect by suppressing and up-regulating certain chemical mediators. This tilts the immune environment toward regeneration (Yin et al., 2022).
Angiogenesis and Tissue Oxygenation
New vessel formation (angiogenesis) is crucial for tissue healing. HBOT boosts endothelial cell activity, accelerating angiogenesis and microvascular recovery (Khan et al., 2009).
Oxidative Preconditioning
Intermittent hyperoxia (higher oxygen) trains the body’s antioxidant defenses. This hormetic stress primes MSCs to tolerate oxidative environments, similar to how strength training builds resilience in muscle (MacLaughlin et al., 2019).
The result is coherence—oxygen and stem cells working in concert to promote healing at a local injury site.
Hormesis is a phenomenon in which low doses of a substance or stressor can have beneficial effects, while higher doses can be harmful. HBOT flirts with the line of just enough before being harmful.
Clinical Applications: Where Science Meets Sport and Longevity
In regenerative medicine, timing is everything. Studies suggest the most effective HBOT protocols for supporting stem cell therapy use:
- Pressure: 2.0–2.5 ATA
- Oxygen Concentration: 100%
- Session Duration: 60–90 minutes
- Frequency: Daily or near-daily for 1–3 weeks
- Timing: Can begin within 24 hours of stem cell administration
Bone and Joint Regeneration
In orthopedic models, HBOT accelerates bone formation, reduces inflammation, and enhances cartilage repair. Animal studies show improved integration of stem cells in osteoarthritis and bone models (Jusković et al., 2022).
Neurological Recovery
Neural tissues are oxygen-sensitive. When combined with stem cells, HBOT improves outcomes in spinal cord and peripheral nerve injury models—reducing inflammation and promoting remyelination (Amiri et al., 2024).
Cardiac Healing
In post-infarct hearts (heart attacks), HBOT enhances transplanted grafts survival and cardiac function recovery (Khan et al., 2009).
Athletic Performance and Recovery
Beyond disease, HBOT offers promise for recovery in athletes. Studies show improved mitochondrial efficiency, reduced inflammation, and faster tissue repair post high-intensity training. When integrated into regenerative programs, HBOT may bridge the gap between recovery and cellular repair—the meeting point of sports science and stem cell biology.
Safety, Contraindications, and Common Misconceptions
Like any therapy that manipulates physiology, HBOT demands precision.
Clinical Guidelines + Potential Risks
2.0–2.5 ATA for 60–90 minutes has been used safely in hundreds of studies with minimal side effects (Gardin et al., 2020; Yin et al., 2022).
- Barotrauma (middle ear or sinus pressure)
- Temporary myopia
- Rarely, oxygen toxicity at >2.8 ATA or prolonged exposure
Soft Chambers vs. Medical Grade Chambers
Consumer-grade “mild” HBOT chambers typically reach only 1.3 ATA—insufficient to replicate the physiological effects seen in clinical research. While they may offer subjective recovery benefits, they likely fail to achieve true tissue hyperoxygenation or stem cell modulation.
The takeaway: pressure matters. Effective HBOT is not about comfort—it’s about dosage and depth.
Evidence-Based Dose and Timing Protocols
From a recent literature review, the following parameters are found:
| Parameter | Optimal Range | Rationale |
|---|---|---|
| Pressure | 2.0–2.5 ATA | Maximizes oxygen diffusion while minimizing oxidative stress |
| Duration | 60–90 min/session | Sustains plasma oxygen saturation beyond red blood cell capacity |
| Frequency | Daily for 7–21 sessions | Enhances cumulative angiogenic and stem cell effects |
| Timing (Post-MSC) | As early as first 24 hours | Aligns with MSC engraftment and early paracrine signaling |
| Caution | >2.8 ATA or >30 sessions | May induce oxidative stress or diminish MSC potency |
In short: the sweet spot is early initiation, moderate pressure, and consistent exposure.
Too little exposure, and the biological cascade doesn’t ignite. Too much, and oxygen becomes corrosive rather than constructive.
Integrating HBOT Into Stem Cell and Performance Therapy: The 3P Model
My 3P Model—Principles, Process, and Plans—provides the structure for integrating HBOT into regenerative and performance programs. Here is how we can do that:
Principles: Grounding in Truth
- Coordinate the System: Oxygen therapy is not just about lungs—it influences circulation, metabolism, and inflammation.
- Force Management: Pressure acts as a mechanical load on the vascular system, demanding regulation of oxidative stress.
- Fitness and Recovery: HBOT amplifies aerobic capacity indirectly through mitochondrial efficiency.
Process: Experiment and Expose
Each athlete or patient is an n=1 experiment. Introduce HBOT as a safe-to-fail exposure, testing individual tolerance and outcome metrics like heart rate variability, recovery time, and perceived experience.
The principle “slow when you can, fast when you must” applies here. Acute injuries may benefit from accelerated exposure post-injection; long-term recovery can favor gradual dosing cycles.
Plans: Control, Measure, Adjust
- Individual Constraints: Consider prior barotrauma, sinus history, and claustrophobia.
- Task Constraints: Match HBOT schedule to the regenerative timeline—post-stem cell therapy suggests early initiation.
- Environmental Constraints: Ensure access to true clinical chambers and medically supervised environments.
Finally, amplify desirables and dampen undesirables: use HBOT to amplify tissue oxygenation, cell survival, and recovery; dampen oxidative stress through spacing and nutrition support (antioxidant-rich diet, polyphenols).
Comparison to Other Recovery Modalities
| Modality | Primary Mechanism | Limitations |
|---|---|---|
| Cryotherapy | Reduces inflammation via vasoconstriction | Limited tissue oxygenation; transient |
| PEMF Therapy | Electromagnetic modulation of cell membranes | Mechanistic overlap but less systemic |
| HBOT (2.0–2.5 ATA) | Hyperoxygenates plasma, mobilizes stem cells, promotes angiogenesis | Requires medical-grade chamber; dosing critical |
Future Outlook
The frontier of regenerative medicine is converging on individualization and precision—not just of cells or molecules, but of oxygen and time.
Emerging research explores HBOT preconditioning before stem cell harvest to increase cell resilience, as well as post-stem cell therapy to enhance recovery.
The message is clear: oxygen is a biologic drug, and like any drug, its efficacy depends on dosage, timing, and context.
Summary and Key Takeaways
- HBOT enhances stem cell function, survival, and differentiation, acting as a biological amplifier.
- The optimal clinical protocol is 2.0–2.5 ATA for 60–90 minutes daily for 1–3 weeks, ideally initiated within 24 hours of MSC therapy.
- Consumer-grade soft shell chambers (≤1.3 ATA) lack sufficient pressure to replicate these effects.
- Integration with the 3P Model ensures HBOT is applied systematically and safely across contexts.
- The future of regenerative therapy lies not in one technology but in synergy—cells, oxygen, and strategy.
5 Questions to Ask Before HBOT and Stem Cell Therapy
- How will nutrition, hydration, and recovery be coordinated to support therapy?
- Is the chamber medical-grade and capable of reaching 2.0–2.5 ATA?
- What is the timing protocol relative to my stem cell procedure?
- How will treatment progress be monitored and adjusted?
- Are there contraindications (e.g., ear issues, respiratory problems)?
Free Download
Read More Like This
Related Podcasts
References
Gardin C, Bosco G, Ferroni L, Quartesan S, Rizzato A, Tatullo M, Zavan B. Hyperbaric Oxygen Therapy Improves the Osteogenic and Vasculogenic Properties of Mesenchymal Stem Cells in the Presence of Inflammation In Vitro. Int J Mol Sci. 2020;21(4):1452. doi:10.3390/ijms21041452
Amiri F, Jafari A, Ahmadi F, Mokhtari H, Raoofi A, Kasmaie F, Omran M, Alimohammadi M, Nasiry D. Exosomes Derived from Human Placental Mesenchymal Stem Cells in Combination with Hyperbaric Oxygen Therapy Enhance Neuroregeneration in a Rat Model of Sciatic Nerve Crush Injury. Regenerative Therapy. 2024;28:30-40. doi:10.1016/j.reth.2024.11.021
Pan HC, Chin CS, Yang DY, Ho SP, Chen CJ, Hwang SM, Chang MH, Cheng FC. Human Amniotic Fluid Mesenchymal Stem Cells in Combination with Hyperbaric Oxygen Augment Peripheral Nerve Regeneration. Neurochem Res.2009;34(7):1304-1316. doi:10.1007/s11064-008-9910-7
Yin TC, Shao PL, Chen KH, Lin KC, Chiang JY, Sung PH, Wu SF, Li YH, Yip HK, Lee MS. Synergic Effect of Combined Therapy of Hyperbaric Oxygen and Adipose-Derived Mesenchymal Stem Cells on Improving Locomotor Recovery After Acute Traumatic Spinal Cord Injury in Rat Mainly Through Downregulating Inflammatory and Cell-Stress Signalings. Cell Transplant. 2022;31:9636897221133821. doi:10.1177/09636897221133821
Jusković A, Nikolic M, Ljujić B, Matic A, Živković V, Vučićević K, Milosavljevic Z, Vojinovic R, Jovicic N, Zivanovic S, Milivojević N, Jakovljevic V, Bolevich S, Kovačević M. Effects of Combined Allogenic Adipose Stem Cells and Hyperbaric Oxygenation Treatment on Pathogenesis of Osteoarthritis in Knee Joint Induced by Monoiodoacetate. Int J Mol Sci. 2022;23(14):7695. doi:10.3390/ijms23147695
Khan M, Meduru S, Mohan IK, Kuppusamy ML, Wisel S, Kulkarni A, Rivera BK, Hamlin RL, Kuppusamy P. Hyperbaric Oxygenation Enhances Transplanted Cell Graft and Functional Recovery in the Infarct Heart. J Mol Cell Cardiol.2009;47(2):275-287. doi:10.1016/j.yjmcc.2009.04.005
Schulze J, Kaiser O, Paasche G, Lamm H, Pich A, Hoffmann A, Lenarz T, Warnecke A. Effect of Hyperbaric Oxygen on BDNF Release and Neuroprotection: Investigations with Human Mesenchymal Stem Cells and Genetically Modified NIH3T3 Fibroblasts as Putative Cell Therapeutics. PLoS One. 2017;12(6):e0178182. doi:10.1371/journal.pone.0178182
Peña-Villalobos I, Casanova-Maldonado I, Lois P, Prieto C, Pizarro C, Lattus J, Osorio G, Palma V. Hyperbaric Oxygen Increases Stem Cell Proliferation, Angiogenesis, and Wound-Healing Ability of WJ-MSCs in Diabetic Mice. Front Physiol. 2018;9:995. doi:10.3389/fphys.2018.00995
MacLaughlin K, Barton GP, Braun R, MacLaughlin JM, Lamers JM, Marcou M, Eldridge MW. Hyperbaric Air Mobilizes Stem Cells in Humans: A New Perspective on the Hormetic Dose Curve. Front Neurol. 2023;14:1192793. doi:10.3389/fneur.2023.1192793
Ko SF, Chen KH, Wallace CG, Yang CH, Sung PH, Shao PL, Li YH, Chen YL, Yip HK. Protective Effect of Combined Therapy with Hyperbaric Oxygen and Autologous Adipose-Derived Mesenchymal Stem Cells on Renal Function in Rodents After Acute Ischemia-Reperfusion Injury. Am J Transl Res. 2020;12(7):3272-3287.
Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem Cell Mobilization by Hyperbaric Oxygen. FASEB J. 2006;20(14):2660-2662. doi:10.1096/fj.06-6649fje
Moniz I, Ramalho‐Santos J, Branco A. Differential Oxygen Exposure Modulates Mesenchymal Stem Cell Metabolism and Proliferation Through mTOR Signaling. Int J Mol Sci. 2022;23(7):3749. doi:10.3390/ijms23073749
Abdelhakim HA, Shune LA, Bhatti SS, Cantilena AM, Lin TL, Ganguly S, Singh AK, Abhyankar S, Lipe BC, McGuirk JP, Allin D, Aljitawi OS. Outcomes of Autologous Hematopoietic Cell Transplantation Patients Receiving Hyperbaric Oxygen Therapy. Biol Blood Marrow Transplant. 2017;23(2):296-302. doi:10.1016/j.bbmt.2016.12.245
Lococo V, Okoniewski M, McGuirk JP, Shune LA, Singh AK, Abhyankar S, Aljitawi OS, Abdelhakim HA. Long-Term Outcomes of Hyperbaric Oxygen Pretreatment in Autologous Hematopoietic Stem Cell Transplantation: A Pilot Clinical Trial. Blood. 2024;143(6):890–900. doi:10.1182/blood-2024-208548
MacLaughlin K, Barton G, Braun R, Eldridge M. Effect of Intermittent Hyperoxia on Stem Cell Mobilization and Cytokine Expression. Med Gas Res. 2019;9(3):139-144. doi:10.4103/2045-9912.266989
Tölle J, Koch A, Schlicht K, Finger D, Kaehler W, Höppner M, Graetz C, Dörfer C, Schulte D, El-Sayed KF. Effect of Hyperbaric Oxygen and Inflammation on Human Gingival Mesenchymal Stem/Progenitor Cells. Cells. 2023;12(20):2479. doi:10.3390/cells12202479
Cheshmi H, Mohammadi H, Akbari M, Nasiry D, Rezapour-Nasrabad R, Bagheri M, Abouhamzeh B, Poorhassan M, Mirhoseini M, Mokhtari H, Akbari E, Raoofi A. Human Placental Mesenchymal Stem Cell-Derived Exosomes in Combination with Hyperbaric Oxygen Synergistically Promote Recovery After Spinal Cord Injury in Rats. Neurotox Res.2023;41(2):431-445. doi:10.1007/s12640-023-00649-0
Jafari A, Khalatbary AR, Taghiloo S, Mirzaie M, Nazar E, Poorhassan M, Akbari E, Asadzadeh M, Raoofi A, Nasiry D. Exosomes Derived from Human Placental Mesenchymal Stem Cells in Combination with Hyperbaric Oxygen Synergically Alleviate Spinal Cord Ischemia-Reperfusion Injury. Regenerative Therapy. 2023;24:407-416. doi:10.1016/j.reth.2023.09.003
Qin-Y W. Clinical Efficacy and Safety of Human Umbilical Cord Mesenchymal Stem Cell Transplantation Combined with Hyperbaric Oxygen Therapy in the Treatment of Traumatic Epilepsy. Hainan Med J. 2013;24(12):1452-1456.
Zhou H, Liu Z, Liu X, Chen Q. Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation Combined with Hyperbaric Oxygen Treatment for Repair of Traumatic Brain Injury. Neural Regen Res. 2016;11(1):107-113. doi:10.4103/1673-5374.175054