Approximate Read Time: 8 Minutes
“Great outcomes start long before the injection—due diligence is the first dose.”
What You will learn
- Verify source, screening, and lab standards.
- Understanding what is a Certificate of Analysis.
- Match delivery method to diagnosis and risk.
Author’s note: This article is educational and not medical advice.
Regenerative medicine sits at the intersection of hope, biology, and regulation. As the space matures, the best clinics are transparent about what they do and how they do it. Others may not be as transparent. This guide distills five crucial questions to ask before you consent to any stem cell therapy—drawn from my ongoing work in regenerative medicine and rehab. For context on how I integrate regeneration into rehab, see Regeneration Meets Rehab: My Journey into Stem Cell Therapies in Panama and Stem Cell Therapy for Athletes: Evidence-Based Healing & Performance Gains.
1) Know Your Ingredients – Stem Cell Source & Donor Screening
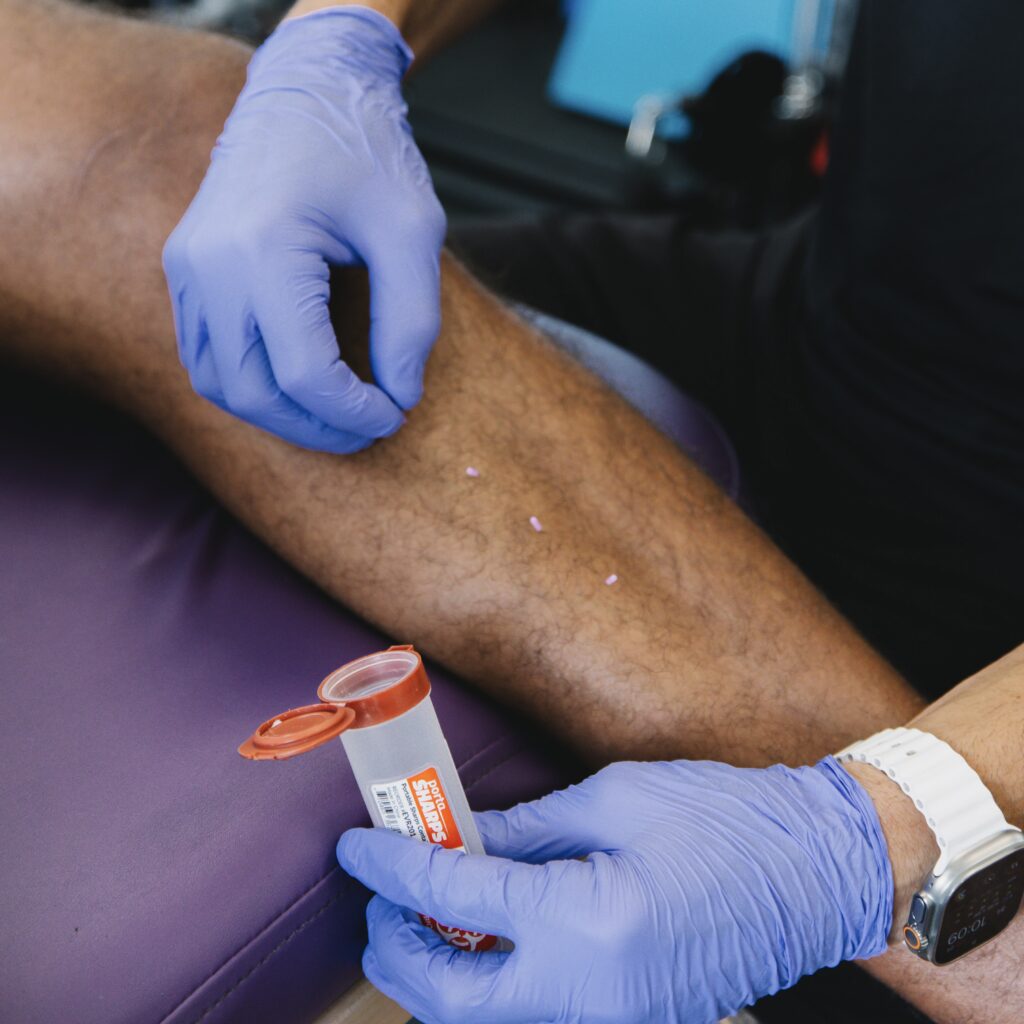
If your body is a high-performance machine, the cells are the parts you’re implanting. You deserve to know exactly where those parts came from and how they were vetted.
Ask plainly: “What is the source (autologous vs allogeneic), what donor screening was performed, and what certifications does the lab hold?”
What good clinics should provide you and what you should hope to learn:
- Source clarity: Autologous (your own bone marrow or adipose) vs allogeneic (donor birth-associated tissues like umbilical cord/Wharton’s jelly). Each path shapes immunologic risk, logistics, and regulatory classification.
- Donor screening & eligibility: Transparent testing for transmissible diseases and medical/social history review; traceability from donor to product lot; documentation that matches the product label.
- Facility standards: For minimally manipulated cells, compliance with Good Tissue Practice (GTP) is expected. For manipulated/expanded cells, Good Manufacturing Practice (GMP) authorization and quality systems are non-negotiable. A credible clinic will name the lab and its accreditation.
Why it matters: risk isn’t just the cell—it’s the chain of custody from donor to you. Global guidance stresses donor screening, establishment registration, and traceability precisely to prevent infectious transmission and ensure recall capability if an issue arises (WHO, 2023).
2) Cell Quality & the Certificate of Analysis
Ask: “Will I receive a lot-specific Certificate of Analysis (CoA) showing identity markers, viability (% live cells) at administration, sterility and endotoxin results, and (if applicable) a potency assay?”
- Identity: Cell surface markers consistent with the product (e.g., MSC immunophenotype if claimed).
- Viability: A percent viability at or near the time of administration; note that post-thaw viability can fall rapidly if handling is sloppy. The higher the number, or closer to 100, the better.
- Safety: Negative tests for microbial contamination (sterility), endotoxin, and mycoplasma; evidence of validated good transport and handling conditions.
- Potency (when applicable): An assay tied to a plausible mechanism (e.g., immunomodulation) rather than a vague “cell count.”
These are basic critical quality attributes for living cell products (WHO, 2023). A clinic that cannot produce a genuine, lot-specific CoA is asking you to fly without instruments.
3) Who’s Watching? Regulatory Oversight, Ethics, and Data Transparency.
Ask: “Under which regulatory pathway is this product being used, and who oversees the clinic?
Clinics operating without regulatory oversight can be a warning sign. The World Health Organization and several other governing bodies exist to support quality control, safety, and efficiency of stem cell treatments. Not having these oversights can potentially lead to lesser quality cells.
Acronyms you may want to be on the lookout for are:
- ISO – International Organization of Standardization
- AABB – Association for Advancement of Blood and Biotherapies
- GMP – Good Manufacturing Practice
4) Route of Administration & Dosing Rationale
Ask: “Why this route (local injection, intra-articular, intradiscal, IV), what imaging guidance is used, and what evidence supports this approach for my diagnosis?”
Understanding the quality of the treatments is the first step. The next step is vetting out the clinical decision making process. Undergoing imaging and medical diagnostics (i.e. labs) is critical to guide what exactly you are going to treat. Blindly receiving treatments without the information beforehand could lead to false expectations, unnecessary treatments, or you may not be optimizing treatments as well. Once you understand what will be treated, then you can understand what delivery method is going to be best.
Below are a few of the most common methods for delivery that I have come across. Intravenous (IV) and intra-articular are the most common and often paired together to support both systemic and local pathways.
- Local vs systemic: For focal musculoskeletal pathology, image-guided local delivery (e.g., ultrasound-guided peritendinous or intra-articular) often makes more mechanistic sense than systemic IV. In a case series on chronic lumbar pain, targeted erector spinae MSC infiltrations were used locally rather than systemically—a strategy aligned with tissue-level intent, though evidence was preliminary (Diaz et al., 2023).
- Guidance & sterility: Ultrasound or fluoroscopy guidance, sterile field, and complication protocols are basic safety layers.
- Dose & schedule: Cell dose, number of passes, and spacing between sessions should be justified—not just “this is what we do.”
5) Expanded or Non-Expanded Cells?
Ask: “Are these cells culture-expanded or minimally manipulated? If expanded, under what GMP conditions and what criteria are used?”
Expanded cells I have come to learn as common practice outside of the United States. The process of expanding cells simply means taking a certain volume of donor cells and growing them in a lab to increase the total number of cells. This allows labs and centers to require less donors, which can be advantageous for the patients as fewer donors are recommended versus multiple donors. Here are a few more quick notes:
- Regulatory line: Culture expansion allows labs to increase the total number of cells from a single donor, but this process triggers higher manufacturing controls, potency testing, and clinical evidence expectations (WHO, 2023).
- Biology changes: Expansion can shift phenotype, so the passage number and culture conditions matter. Over-expansion risks poorer quality cells.
Smart Add-Ons: Two Questions You Might Not Think to Ask
A) What’s the role of exosomes here—if any?
Some clinics market exosomes as a cell-free alternative or adjunct. Exosomes are secreted by MSCs, therefore if you receive cells you may not need the exosomes. Many centers will combine the two to enhance the regenerative properties of the stem cells.
B) How will rehab, load management, and fitness be integrated post-procedure?
Cells don’t lift weights for you. A high-quality clinic will map your return to loading and conditioning using objective checkpoints instead of time alone. This is where regeneration meets the 3P Model—principles, process, and plans—so biology and behavior move in lockstep.
Putting It Together
- Source & screening: Donor eligibility, traceability, and lab accreditation.
- Quality proof: Lot-specific CoA with identity, viability, sterility, and (when appropriate) potency.
- Oversight & data: Clear regulatory pathway, ethics review, and adverse-event reporting.
- Delivery logic: Route matches pathology; image guidance and sterile technique documented.
- Manipulation level: Expanded vs non-expanded—with GMP manufacturing and release criteria if expanded.
For a deeper primer, listen to Stem Cells, Exosomes, Peptides, and Orthobiologics: A Masterclass with Dr. Chuck Peterson and Dr. Cameron Davis and cross-reference concepts with the articles linked throughout.
Conclusion
When you’re evaluating stem cell therapy, the biggest variable isn’t the brand name on the clinic door or influencer promoting them—it’s the process behind it. Ask about source, quality, oversight, delivery, and expansion. Require documentation (CoA), not just confidence. Match the tool to the tissue, and insist that biology is integrated with a measured, data-informed rehab plan.
Read More Like This
Related Podcasts
References
Diaz C, Sandoval L, Ramirez M, Utley J, Briggs D. Erector spinae infiltration with mesenchymal stromal cells in chronic lumbar pain: a case report series. Genesis Journal of Medicine and Medical Research. 2023;4(1):17-23. https://www.genesispub.org/erector-spinae-infiltration-with-mesenchymal-stromal-cells-in-chronic-lumbar-pain-a-case-report-series
Briggs D, Utley J. Advancements in Athlete Injury Repair: The Significance of Stem Cell Therapy in Musculoskeletal Healing. Panama City, Panama: Auragens Publishing; 2024.
Briggs D, Utley J. Exosomal Elixir: Unleashing the Power of Mesenchymal Stem Cell-Derived Exosomes in Regenerative Medicine. Panama City, Panama: Auragens Publishing; 2024.
World Health Organization Expert Committee on Biological Standardization. Considerations in developing a regulatory framework for human cells and tissues and for advanced therapy medicinal products. Adopted March 20–24, 2023. (Final edited version for WHO Technical Report Series). (Cited inline and discussed with reference to Appendix 1 and Appendix 2).