“Just like you need protein intake with resistance training to build muscle, you need stem cells with physical therapy to repair degenerative tissues.”
Adam Loiacono
What You will learn
- Stem cells can enhance rehab outcomes, especially in degenerative injuries.
- Umbilical cord-derived MSCs are ethical, potent, and regenerative.
- Quality and viability of stem cells matter greatly.
- Stem cells are a complement—not a replacement—for physical therapy.
- Misconceptions in the U.S. often limit access to effective regenerative treatments.
New Chapter Begins – Panama City with Auragens
For the past 15 years, I’ve been immersed in the world of professional sports, helping athletes—from NBA All-Stars to World Cup champions—get out of pain and back to performance. But recently, I found myself in a completely different setting: Panama City, Panama.
Not for vacation. For evolution.
I spent a week collaborating with a group of pioneering doctors at Auragens, a clinic on the cutting edge of regenerative medicine. The team included:
- Dr. Dan Briggs – an expert in orthopedic and regenerative protocols
- Dr. James Utley – a leader in stem cell applications
- Dr. Christian Diaz – clinical research in mesenchymal stem cell therapies
Together, we supported a client through a comprehensive regenerative protocol that combined:
- Intravenous umbilical cord mesenchymal stem cells (UC-MSCs)
- Intra-articular joint injections for degenerative conditions
- Exosomes and platelet-rich plasma (PRP) to target inflammation and repair


But this trip was about more than just new tools—it reframed the way I see physical therapy itself. It reminded me that in order to help our clients reach their full potential, we must embrace new science, new strategies, and new partnerships.
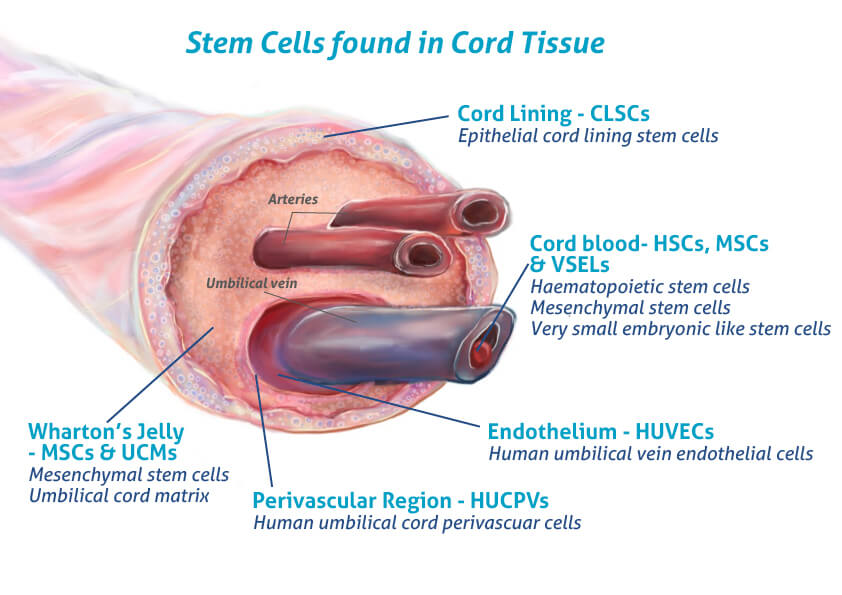
What Are Umbilical Cord-Derived Mesenchymal Stem Cells (UC-MSCs)?
Stem cells can sound intimidating or even controversial. But let’s get something clear:
These cells come from the umbilical cord—tissue that would otherwise be discarded after birth. No embryos. No controversy. 100% ethical.
Mesenchymal stem cells (MSCs) are multipotent stromal cells that can:
- Differentiate into bone, cartilage, muscle, and fat cells
- Modulate the immune system
- Decrease inflammation
- Support tissue regeneration and healing
And when they come from the umbilical cord, they are:
- Younger and more robust than adult MSCs (from bone marrow or adipose tissue)
- Naturally immune-privileged, reducing rejection risk
- High in paracrine signaling, releasing healing signals even without becoming new tissue
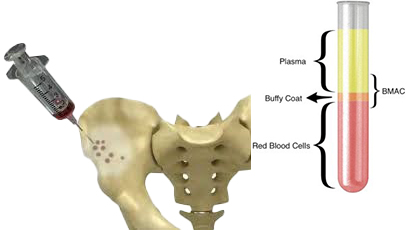
But it’s not just about the source. Quality matters.
Why Viability > Quantity
According to World Health Organization (WHO) standards, a stem cell product should be at least 75% viable. That means 75% of the cells are alive and functional when administered.
Unfortunately, not all clinics meet this standard—and patients pay the price with low-grade therapies and poor outcomes.
At Auragens, the products used meet or exceed WHO guidelines, ensuring:
- High cell viability
- Stringent testing protocols
- Transparent lab certifications
As a physical therapist, this matters. Just like protein supports strength training, stem cells enhance the outcomes of rehab—but only if the ingredients are clean, viable, and responsibly sourced.
Myths, Misconceptions & The U.S. Disconnect
In the United States, the landscape of stem cell therapy is… complicated.
Many patients are skeptical. Rightfully so. There’s a fog of misinformation and “stem cell mills” that cloud the real science. The lack of robust clinical trials, success stories, and unsuccessful stories can really make for a challenge to find value.
Here are the 5 most common myths I hear:
Myth 1: “Stem cells come from embryos.”
False. Most clinical regenerative treatments today use adult or perinatal stem cells, including:
- Bone marrow
- Fat tissue
- Umbilical cords
No embryos involved.
Myth 2: “Stem cell therapy is illegal in the U.S.”
Partially true. The FDA restricts expanded or cultured stem cells, but same-day procedures using autologous tissue are legal. Unfortunately, this limits access to the more powerful, lab-prepared therapies available internationally.
Myth 3: “Stem cells replace surgery.”
Not always. While stem cells may help delay or avoid surgery, especially for cartilage or tendon injuries, they aren’t a substitute for all orthopedic interventions.
Myth 4: “There’s no science behind it.”
Emerging research is growing. Case studies, imaging, and even blinded trials are beginning to show significant regenerative effects, particularly in orthopedic tissues.
Myth 5: “It’s a scam.”
Bad actors exist. But that’s also true in fitness, supplements, and yes—conventional medicine. The key is vetting the provider, the product, and the purpose.
Case Spotlight – Erector Spinae MSC Infiltration
One of the most exciting studies to come from this team is a case report led by Dr. Christian Diaz, published in Genesis Publications.
Study: Erector Spinae Infiltration with Mesenchymal Stromal Cells in Chronic Lumbar Pain: A Case Report Series
In this study, patients with chronic lumbar pain received ultrasound-guided MSC injections into the erector spinae muscles—a notoriously difficult area to manage through manual therapy or injections.
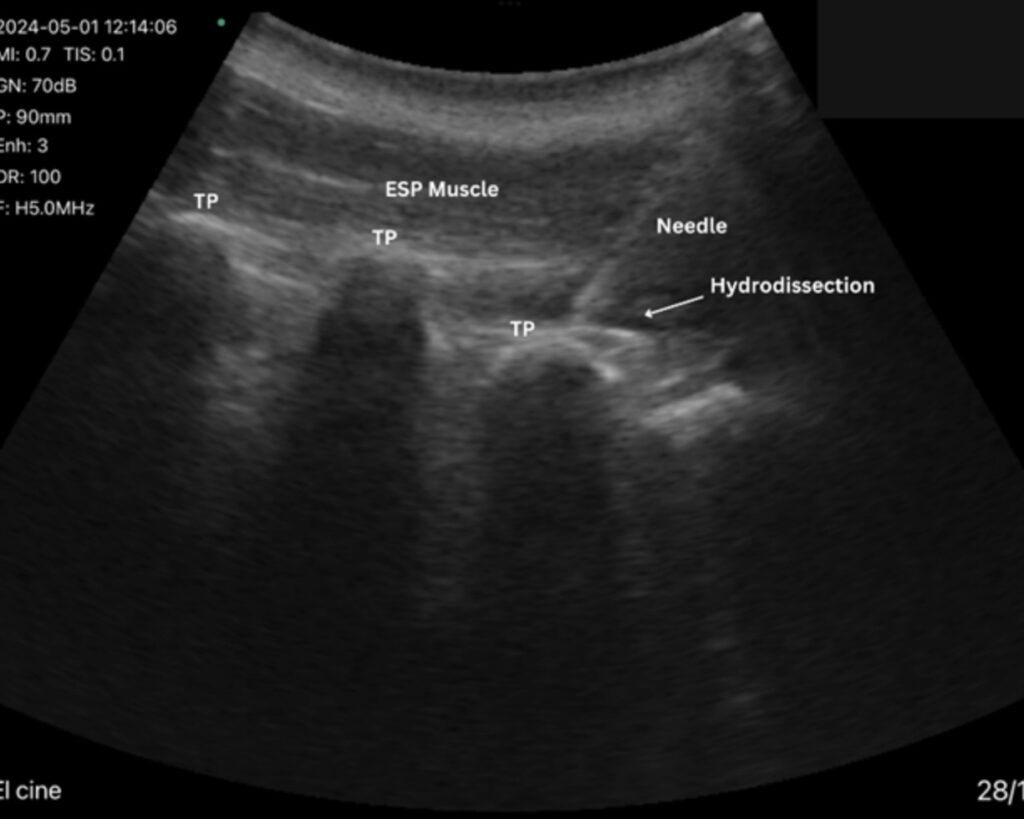
Key outcomes
- Pain reduction measured by VAS
- Improved function via physical performance tests
- Sustained benefit up to 6 months post-injection
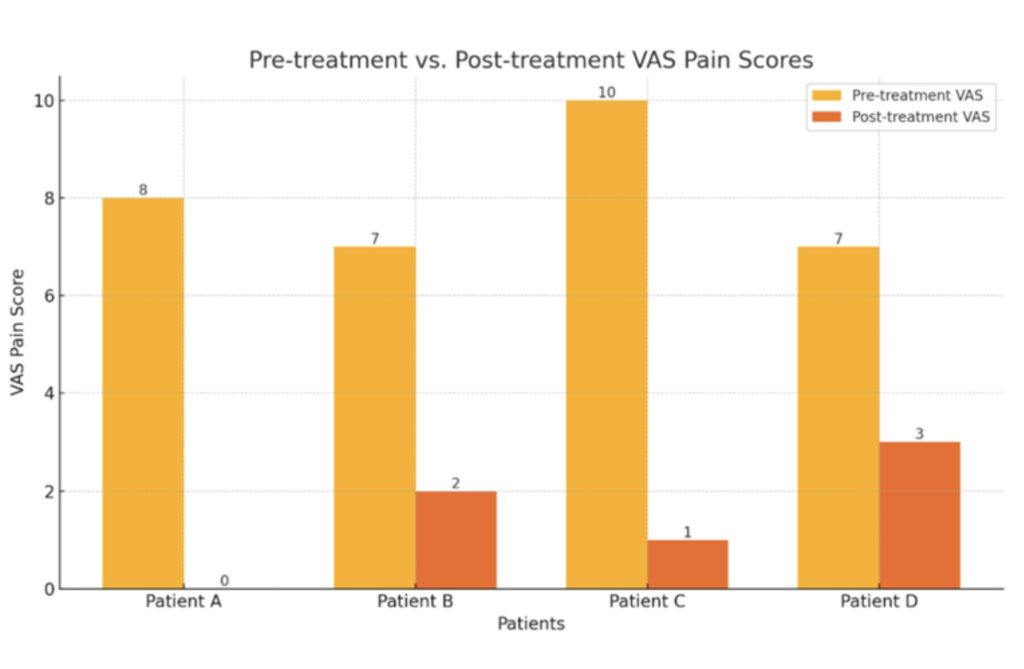
This is a perfect example of how manual therapy or exercise alone may not solve every problem—but in conjunction with targeted biological inputs, we create an environment for healing that no single modality can achieve.
Current Limitations, Cost, and Considerations
Let’s be clear. Stem cells are not magic. They’re a tool, not a cure-all.
Current Challenges:
- Limited large-scale clinical trials – Most data is case-based or from small studies
- Variability in regulation – FDA restrictions vs. international practices
- High cost – Treatments range from $8,000–$25,000 depending on protocol
- Travel burden – Most advanced therapies require international travel
What This Means for Patients and Physical Therapists:
- Do your homework. Vet the clinic.
- Know that results can vary.
- Set realistic expectations.
- View it as a complement to your training, rehab, or recovery plan—not a standalone solution.
Stem Cells + PT = A New Standard
Here’s the bottom line:
“Just like you need protein intake with resistance training to build muscle, you need stem cells with physical therapy to repair degenerative tissues.”
As physical therapists, we’ve been fighting an uphill battle with degenerative injuries:
- Chronic cartilage loss
- Persistent tendinopathies
- Post-surgical inflammation
Sometimes, despite all the manual therapy, strength training, and movement education, results plateau. That’s where regenerative medicine steps in.
Professional athletes are doing it. So are CEOs, veterans, and active adults looking to stay in the game.
The Call for Our Profession
I’m not saying stem cells are for everyone. I’m not even saying they work every time.
But I am saying it’s time we open the door and walk through it together.
If you’re a PT, ATC, chiropractor, or S&C coach—start learning. If you’re a patient—start asking. If you’re curious—start exploring.
This is only the beginning. And I’m just getting started.
Learn from top sports docs
Arizona’s top sports docs join me in this conversation to explore the world of stem cells and other orthobiologics.

insightful videos
Subscribe today to Adam’s YouTube channel to get the latest on rehab, training, sports science, and so much more!
References
Diaz C, Sandoval L, Ramirez M, Utley J, Briggs D. Erector spinae infiltration with mesenchymal stromal cells in chronic lumbar pain: a case report series. Genesis Journal of Medicine and Medical Research. 2023;4(1):17-23. https://www.genesispub.org/erector-spinae-infiltration-with-mesenchymal-stromal-cells-in-chronic-lumbar-pain-a-case-report-series
Briggs D, Utley J. Advancements in Athlete Injury Repair: The Significance of Stem Cell Therapy in Musculoskeletal Healing. Panama City, Panama: Auragens Publishing; 2024.
Available on Amazon
Briggs D, Utley J. Exosomal Elixir: Unleashing the Power of Mesenchymal Stem Cell-Derived Exosomes in Regenerative Medicine. Panama City, Panama: Auragens Publishing; 2024.
Available on Amazon
World Health Organization. Flow cytometry study report for mesenchymal stromal cells. 2019.
Available at: https://cdn.who.int/media/docs/default-source/biologicals/ecbs/reference-materials/bs-2019-2376-msc-flow-cytometry-study-report-ecbs-final.pdf
U.S. Food & Drug Administration. FDA regulation of human cells, tissues, and cellular and tissue-based products (HCT/Ps). 2020.
Available at: https://www.fda.gov/vaccines-blood-biologics/tissue-tissue-products